 |
| Class 10 syllabus 2021-22 |
Notes of caustic soda(NaOH)
Caustic soda is an alkali salt which is also called Lye. It is the common name of sodium hydroxide. This name is given due to the corrosive nature of this salt on animal and plant tissues. It has a wide range of applications. The chemical formula of sodium hydroxide is NaOH.
Method of preparation
When electricity is passed through an aqueous solution of NaCl (brine).it decomposes to form NaOH,this process is called chlor alkali process.
2NaCl +2H2O------>2NaOH + 2H2+Cl²
Properties of Caustic Soda
1.It is a white solid which has a melting point of 591K
2.It is a stable compound.
3.NaOH is bitter and has a soapy feel to it.
4.It is highly soluble in water and moderately soluble in alcohol.
5.Sodium hydroxide is strongly alkaline in nature.
Caustic Soda Uses
1.It is used as a cleansing agent and in the manufacturing of washing soda.
2.Sometimes, sodium hydroxide is also used as a reagent in the laboratories.
3.It is used in the preparation of soda lime.
4 It is used in the extraction of aluminium by purifying bauxite.
2. Why do we call Sodium Hydroxide as Caustic Soda?
Ans: Sodium hydroxide is also called soda or lye, or even caustic soda. Here, caustic means' burning', and the caustic soda takes its name the way it can burn the skin, and it has a chemical formula of NaOH.at room temperature, the Sodium hydroxide or Caustic Soda is a white-colored crystalline odorless solid that absorbs moisture from the air, and it is a synthetically manufactured substance. We can say it is a versatile alkali. It is mainly used in the manufacturing of paper, soap & detergents, alumina, petroleum & chemical products. A few other applications include textiles, water treatment food, mining glass making, metal processing.
BLEACHING POWDER(CaOCl2)
- Bleaching powder is a pale yellowish powder existing with a strong smell of chlorine.
- It is soluble in water but due to the presence of impurities, we never observe a clear solution.
- Its chemical formula is
Ca(OCl2) with its chemical name as Calcium hypochlorite. 1. Preparation of Bleaching Powder
Bleaching powder is synthesized by the action of chlorine gas (produced from the chlor-alkali process) on dry slaked lime (
Ca(OH)2 ).Ca(OH)2 + Cl2 → Ca(OCl2) + H2O - properties
- when bleaching powder is treated with an excess of dilute sulphuric acid, all the chlorine present in it liberated:
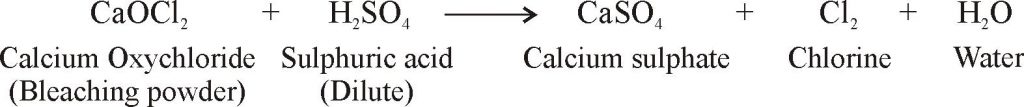
- Here, the chlorine produced by the action of dilute acid on bleaching powder acts as a bleaching agent. Thus, the real bleaching agent present in bleaching powder is chlorine.
2. Uses of Bleaching Powder
- It is used for bleaching dirty clothes in the laundry, as a bleaching agent for cotton and linen in the textile industry.
- It is a strong oxidizing agent, hence used as an oxidizer in many industries.
- It is used as a disinfectant which is used for disinfecting water to make potable water.
- It is used for making wool un-shrinkable.
Note: Exposure to bleaching powder can be dangerous or even fatal if it mixes with other household chemicals. For example, when combined with ammonia, bleaching powder will create a toxic gas called chloramines.
Baking soda(NaHCO3)-
It is also called sodium bi carbonate. It was in the year 1846, John Dwight and Austin Church started a manufacturing unit to produce baking soda using sodium carbonate and carbon dioxide.
Preparation of Baking Soda
Solvay process is used for the production of sodium bicarbonate and sodium carbonate industrially. In this process carbon dioxide, water, ammonia and brine solution in its concentrated form, are used as raw materials. This process is used mainly because it is inexpensive and less number of raw materials are used to produce necessary chemicals. The important chemical reaction that is used in the production of baking soda and sodium carbonate is:
CO2 + H2O + NH3 + NaCl → NaHCO3 + NH4Cl
Properties of Sodium Bicarbonate
- It is non-flammable.
- Powder dust is not as explosive.
- It has a melting point of 500
- NaHCO3 is a white crystalline solid which is odourless.
- It is basic in nature.
- when baking soda is heated it decomposes to give sodium carbonate.
- 2NaHCO3-------->Na2CO3 +H2O +CO2
Uses of Baking Soda
- Reduces the acidity in the stomach
- Acts as an antacid which is used to treat stomach upset and indigestion
- Used in the process of washing as a water softener
- Due to the formation of soapy foam, it is used in fire extinguishers
- Removes the dirt off materials without damaging the properties of the material
- Acts as a pesticide
- Used in baking industries as carbon dioxide is generated (due to the decomposition of NaHCO3) which helps in the raising of the dough.it is mix with mild edible tartaric acid to make its taste better.
- It is used in ear drops, cosmetics and personal care products
- It is used as a neutralizer to neutralize the effect of acid
Washing soda(Na2CO3.10H2O).
Washing soda finds its application in numerous ways, be it from household uses to a vast range of industrial applications. It is an alkaline compound with a high alkaline character which has the capability to remove adamant stains from clothes during washing. Washing soda formula is written as Na2CO3.10H2O. The chemical name of washing soda is sodium carbonate. Chemically soda ash is a hydrated salt of sodium carbonate.
Solvay Process- Preparation of Sodium Carbonate
Steps involved in the manufacture of sodium carbonate are explained below:
- Purification of Brine
- Formation of sodium hydrogen carbonate
- Formation of sodium carbonate
- Recovery of ammonia
Step 1: Purification of Brine
Concentrated brine is obtained by the process of evaporation and impurities like calcium, magnesium, etc are removed by the precipitation process. The concentrated brine solution undergoes filtration and is mixed with ammonia in the ammonia tower and the ammonia tower gets cooled.
Step 2: Formation of sodium hydrogen carbonate
In a carbonate tower, carbon dioxide is passed through an ammoniated brine solution.
NH3(aq) + CO2(g) + NaCl(aq) +H2O → NaHCO3(s) + NH4Cl(aq)
Step 3: Formation of sodium carbonate
Sodium Bicarbonate (NaHCO3) formed is obtained from the tower and is heated at a temperature of 300°C. Hence, formations of sodium carbonate take place.
2NaHCO3 → Na2CO3 + CO2 + H2O
Step 4: Recovery of ammonia
Ammonia can be recovered by treating the solution of NH4Cl with Ca (OH)2. This ammonia is again used in the Solvay process and CaCl2 is obtained as a by-product.
2NH4Cl + Ca(OH)2 → 2NH3 + CaCl2 + H2O
Physical properties and chemical properties of sodium carbonate:
- It is a crystalline solid which is white.
- It exists as a monohydrated salt (Na2CO3.10H2O), anhydrous salt (Na2CO3), heptahydrous salt (Na2CO3.7H2O) and decahydrate salt (Na2CO3.10H2O).
- Sodium carbonate is basic in nature.
- It has a melting point of 851°C.
- In the presence of heat, it loses its water to form an anhydrous salt (soda ash).
Na2CO3.10H2O → Na2CO3.H2O → Na2CO3 (at 373 K)
Uses of Washing Soda:
- Used as a cleansing agent in industries and household.
- It finds its application in paper, textile, soap, and detergent industries.
- It is used in the process of softening of water.
- It is used in the manufacturing of glass.
- It is one of the most important agents in laundries.
.Plaster of paris(P.O.P)----->
What is Plaster of Paris?
Plaster of Paris is a popular substance that is utilized most commonly for sculpting materials and in gauze bandages. While we have seen many applications of this material in our everyday lives if we try to understand its chemistry, plaster of Paris is a white powdery chemical compound which is hydrated calcium sulfate that is usually obtained from calcining gypsum.
Plaster of Paris is also referred to as Gypsum plaster. The chemical formula of plaster of Paris is written as CaSO4·H2O or 2CaSO4·H2O.
Preparation of Plaster of Paris
Plaster of Paris is manufactured by heating gypsum at 423K or 150o C/300o F.
CaSO4·2H2O + heat → CaSO4·0.5H2O + 1.5H2O (discharged as steam)
On heating gypsum at 423 K, it loses water molecules and becomes calcium sulfate hemihydrate. This product is known as the plaster of Paris. However, when water is mixed with dry plaster of Paris, it re-structures into gypsum. As for the process of hardening and setting, it starts around 10 minutes subsequent to blending and is completed in about 45 minutes. It is not completely set for 70-75 hours.
On the other hand, when plaster or gypsum is heated at temperatures higher than 266 °F (130 °C), then we obtain hemihydrate. Other compounds are also formed when gypsum is formed at different temperatures. For example;
When it is heated to about 180 °C, γ-anhydrite is formed. Similarly, when it is heated above 250 °C, β-anhydrite or dead burned plaster is formed and it is a completely anhydrous product.
Characteristics of Gypsum Plaster
Plaster of Paris is usually a white dry plaster powder. It can be effectively worked with metal apparatuses or even abrasive sheets and can be shaped as per the requirements. The strength of plaster of Paris is not as strong as other compounds and it often requires external support when a large amount is used. It is often applied in the form of a quick-setting paste with water
Plaster of Paris Uses
Usually, there are different categories of plaster of Paris and they have different applications. We will further look at some of the most common uses below.
In Architecture and Decorations
Plaster of Paris is utilized to make fine artwork for decoration and beautification of monuments and buildings. These might be geometric (imitating natural rocks and temples) or inspired by nature (like flowers or forests). These are also frequently used to imitate wood or stone mostly found in ancient buildings and monuments.In the present day, this material is often utilized for false ceilings. In this, the powder of plaster of Paris is changed into a sheet structure and then these sheets are fixed to roofs.
In Art
Majority of the great classical wall painting works of art in Europe, similar to Michelangelo’s Sistine Chapel ceiling, are executed in fresco, which means they are painted on a fine layer of wet plaster, called intonaco; the colours sink into this layer in such a way that the plaster itself turns into the medium holding them, which results in the brilliant strength of fresco. Extra work might be included a secco top of the dry plaster, however, this is commonly less tough.
Plaster (also called stucco in this situation) is a far simpler material for making reliefs than stone or wood and was generally utilized for the building walls reliefs in Egypt and the Near East.
During Burial Services
Plaster is utilized by numerous morticians and executives of funeral houses to remake the damaged tissue, rejoin cut off parts of dead bodies, and fill wounds that occurred.
Medicinal Purposes
In the medical field, Plaster of Paris is still frequently utilized a mould and casts. Plaster of Paris is generally utilized as a plaster to join broken bones; a bandage soaked with plaster is added to water and afterwards folded over the broken part of the body, setting into a protective and supportive coating, known as an orthopaedic cast.
Fireproofing
Many fireproofing products and fire protection systems make use of plaster of Paris. The plaster coating discharges water vapours when the building catches fire and thus helping to retard the spread of the fire. It also gives some protection to slow down the heat circulation into steel and concrete components, that would lose their strength and breakdown in a fire.
3D printing
Gypsum plaster is also used in 3D printing nowadays, where the water is specifically applied by the inkjet head.







No comments:
Post a Comment